Topic: Advancing Treatment-Free Remission (TFR) in CML Worldwide
Date: December 6, 2024 | Location: San Diego (USA)
Meeting chair: Dr Katia Pagnano, HEMOCENTRO / UNICAMP – Brazil)
The 2024 iCMLf Forum focused on advancing Treatment-Free Remission (TFR) for CML globally. Expert speakers shared progress in research, diagnostics, and strategies for enhancing TFR access, particularly in low- and middle-income countries (LMICs).
 Session 1: The iCMLf – Planning for the future
Session 1: The iCMLf – Planning for the future
Speaker: Nicola Evans, Chief Executive, iCMLf
Nicola Evans provided an overview of iCMLf’s mission, vision and future direction, emphasising the continued support for equal access to treatment, diagnostics, and care for all CML patients.
The iCMLf leadership is finalising the strategies and projects for the next 10 years.
This will focus on:
- Expanding CML education globally with a dedicated focus on LMICs
- Increasing access to diagnostics
- Providing overarching global patient support and information
- Continuing to research optimal treatment pathways
More details to follow in early 2025…
Session 2: Understanding the Optimal Pathway to Successful TFR
 Presentation: Biological Barriers to Cure
Presentation: Biological Barriers to Cure
Speaker: Professor Brian J. Druker, OHSU Knight Cancer Institute, USA
Professor Druker discussed intrinsic and extrinsic barriers preventing TFR and proposed research strategies to overcome them.
Key Points:
- Barriers to achieving TFR:
- Cell-intrinsic factors: Kinase-independent survival pathways, epigenetic reprogramming, and metabolic adaptations allow residual leukemia cells to persist.
- Cell-extrinsic factors: Immune system interactions, inflammatory responses, and the bone marrow niche play critical roles in maintaining minimal residual disease (MRD).
- Long-term patient management:
- Achieving deep molecular response remains a priority, with current therapies enabling TFR in only 10-20% of patients.
- Shared decision-making is vital for managing treatment cessation, balancing benefits (e.g., reduced toxicity) with potential relapse.
- Strategic goals for future therapies:
- Targeting residual leukemic stem cells through safe, precise therapies.
- Utilising global sample repositories to identify novel therapeutic targets and biomarkers for TFR success
Presentation: Innovative Biomarkers for TFR
Professor Ong presented data on biomarkers predicting TFR success and relapse, using single-cell technologies to identify stem cell differences.
Key Points:
- Role of leukemic stem cells (LSCs):
- Pre-treatment biology at the LSC level determines TFR outcomes, with specific biomarkers predicting relapse or sustained remission.
- Patients with high LSC burden or certain genetic signatures exhibit higher risk of relapse post-TKI cessation.
- Predictive biomarkers:
- Rapid BCR::ABL1 decline at three months is linked to long-term TFR success, suggesting favourable pre-existing stem cell biology.
- Identification of cell surface protein biomarkers (e.g., in CD34+ cells) at diagnosis may enable patient stratification for TFR attempts.
- Single-cell technologies:
- Leveraging single-cell RNA sequencing to map stem cell states, highlighting proliferative pathways (e.g., IL-2/STAT5 signalling) in relapsed patients.
- Epigenetic gene sets in relapsed LSCs provide potential therapeutic targets for future interventions.
Session 3: Successful TFR Globally
 Presentation: TFR in Low-and-Middle-Income Countries
Presentation: TFR in Low-and-Middle-Income Countries
Professor Akhil Ranjon Biswas, Dhaka Medical College, Bangladesh (presented by Dr Shanmuganathan)
Insights on TFR in LMICs, including how this can be achieved, patient perceptions and the challenges of limited access to TKIs, testing, and monitoring infrastructure.
Key Points:
- Access barriers in LMICs:
- Limited availability of TKIs due to lack of insurance and reliance on out-of-pocket expenses.
- Variable access to molecular testing with concerns over sensitivity, reliability, and high costs.
- Practical monitoring solutions:
- Use of FISH cytogenetics and qualitative PCR as cost-effective alternatives to quantitative BCR-ABL testing.
- Reducing test frequency while maintaining safety, e.g., bimonthly testing in the first six months.
- Improving patient compliance:
- Health literacy programs to educate patients on the importance of adherence and timely molecular monitoring.
- Strengthening government support for free TKIs and subsidised diagnostics.
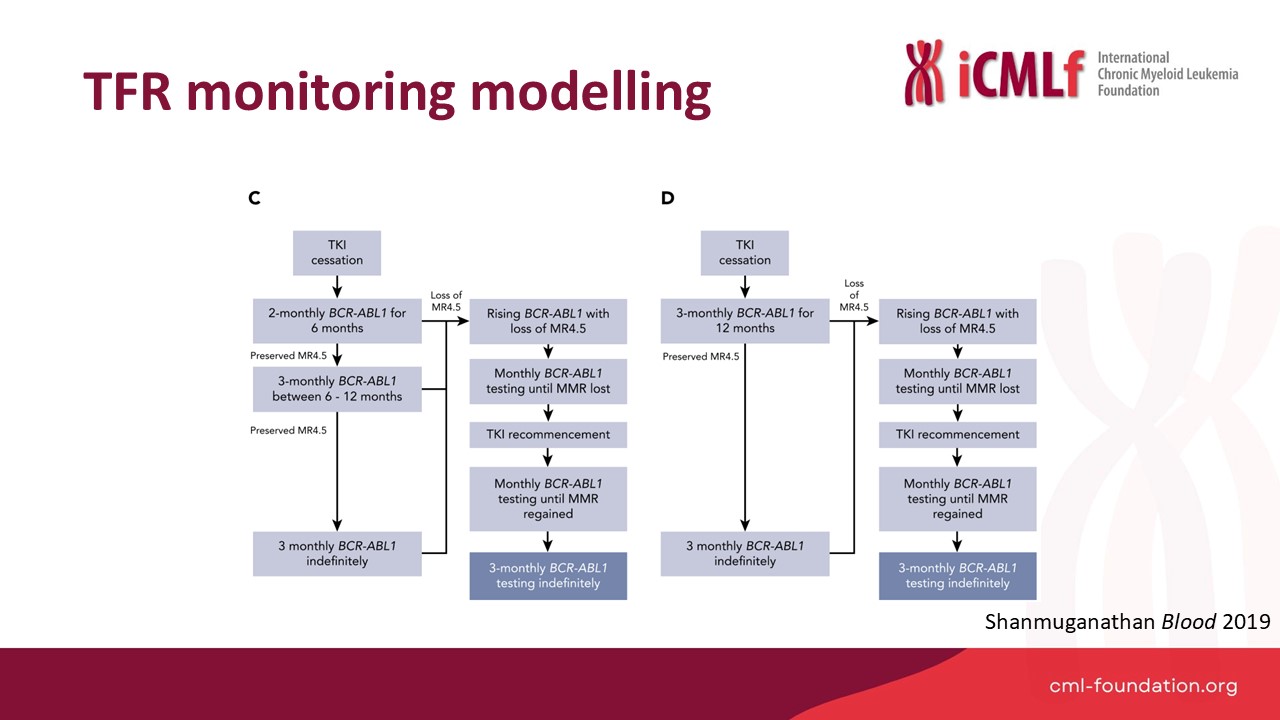 Presentation: Optimal TFR Monitoring
Presentation: Optimal TFR Monitoring
Speaker: Dr Naranie Shanmuganathan, Royal Adelaide Hospital, Australia
Dr Shanmunagathan presented optimised TFR monitoring strategies and practical approaches to improve global accessibility.
Key Points:
- Optimised monitoring protocols:
- Modelling reduced testing regimes (e.g., monthly for six months, then bi-monthly) showed significant cost reductions while maintaining safety.
- Real-world implementation of updated NCCN and ELN guidelines would reduce testing burden.
- Global disparities:
- Successful TFR programs rely on reliable, timely, and affordable BCR::ABL1 testing infrastructure.
- Regional and national efforts must address gaps in testing capabilities for widespread adoption of TFR strategies.
 Presentation: Expanding Diagnostics for CML
Presentation: Expanding Diagnostics for CML
Speaker: Professor Jerry Radich, Fred Hutchinson Cancer Center, USA
Professor Radich highlighted novel diagnostic solutions, including portable PCR devices, lateral flow assays, and nanopore sequencing for LMICs.
Key Points:
- InnovativeV diagnostic tools:
- Development of electricity-free PCR and lateral flow assays as affordable, portable options for LMICs.
- Digital PCR offers superior sensitivity compared to conventional RT-PCR for detecting MRD and predicting relapse.
- Advances in sequencing technologies:
- Portable nanopore sequencing provides real-time results, enabling genetic analysis without reliance on centralised facilities.
- Integration of sequencing data into mobile applications allows for rapid diagnostics and results sharing in remote areas.
- Future directions:
- Use of dried blood spot technology for multiplexed testing, including resistance mutations and whole-genome panels.
- Expanding programs like “Spot on CML” to support large-scale diagnostics globally.
Panel Discussion
Moderator: Giora Sharf, CML Advocates Network
Panellists shared insights on the challenges and future of TFR, emphasising practical, affordable solutations for LMICs.
Key Topics:
- Balancing monitoring rigor and accessibility:
- Flexible protocols (e.g., reduced testing frequency) are necessary for resource-limited settings without compromising safety.
- Long-term monitoring beyond five years:
- Shared decision-making with patients is critical, with proposals for six-monthly or annual testing based on individual risk.
- Practical TFR implementation in LMICs:
- Strategies to increase government-funded TKIs and diagnostics, alongside education programs to improve compliance and patient outcomes.
Conclusion
The 2024 iCMLf Forum delivered actionable insights to advance TFR worldwide. Experts emphasised collaboration, innovation, and equity in addressing barriers to care, particularly in LMICs, with a shared vision of achieving a cure for CML.










