CML paper summary: High-throughput drug screening identifies SMAC mimetics as enhancers of NK cell cytotoxicity in chronic myeloid leukemia (Nygren P. et al. Blood, January 2025)
CML paper summary - April 2025
High-throughput drug screening identifies SMAC mimetics as enhancers of NK cell cytotoxicity in chronic myeloid leukemia
Nygren P. et al. Blood, January 2025
Link to full paper: https://doi.org/10.1182/blood.2024025286
Summary
This study explored how natural killer (NK) cells, a type of immune cell known for targeting cancer cells, could be more effective against chronic myeloid leukemia (CML) when supported by specific drugs. Using a high-throughput drug screening of over 500 small-molecule compounds, the researchers evaluated the impact of oncological drugs on NK cell-mediated cytotoxicity against CML cells.
Introduction
CML is a myeloproliferative disorder driven by the BCR::ABL1 fusion gene. Although tyrosine kinase inhibitors (TKIs) have revolutionized treatment, many patients fail to achieve long-term treatment-free remission, often due to the persistence of leukemic stem cells. NK cells have the potential to control residual disease and achieve remission. However, the efficacy of NK cell-based immunotherapy in CML has not been demonstrated. This study was conducted to identify drugs that enhance NK cell cytotoxicity and better understand the molecular mechanisms behind NK cell-mediated anti-leukemia activity.
Study methods
The researchers employed a high-throughput drug screening approach using a library of 527 oncological compounds. The screening involved co-culturing NK cells with K562 leukemia cells, with target cell viability assessed via a luciferase-based assay. Differential drug sensitivity scores (dDSS) were calculated to quantify the effects of each drug on NK cell-mediated killing.
Subsequent experiments validated top hits using flow cytometry and colony-forming assays on both cell lines and primary CML patient samples. The researchers also utilized single-cell RNA sequencing to profile drug-induced transcriptomic changes in NK and target CML cells, identifying shifts in activation states and gene expression pathways.
Key findings
- SMAC mimetics identified as enhancers: SMAC mimetics birinapant and NVP-LCL161 significantly increased NK cell cytotoxicity in both cell lines and patient-derived CML samples.
- Negative impact of certain drugs: Glucocorticoids (e.g., dexamethasone) and some TKIs (e.g., dasatinib) suppressed NK cell activity and IFN-γ production.
- Single-cell insights: Drug-induced changes in NK and CML cell transcriptomes revealed enhanced NF-κB signalling with SMAC mimetics and inhibition of activation markers with suppressive drugs.
- Clinical implications: The findings support the potential of SMAC mimetics in combination NK cell immunotherapy, especially for advanced-phase CML or TKI-resistant cases.
Conclusions
This research presents a promising strategy for enhancing NK cell-based immunotherapy in CML. The identification of SMAC mimetics as potent enhancers of NK cell cytotoxicity offers a foundation for clinical studies and suggests broader applications in other malignancies. Further preclinical and clinical studies are needed to assess safety, efficacy, and optimal treatment combinations.
Further commentary:
SMAC mimetics in action in chronic myeloid leukemia
Ali G. Turhan. Blood, April 2025
https://doi.org/10.1182/blood.2024027994
Overview of clinical trials in CML - Not yet recruiting trials
New CML compounds in development
Source: www.clinicaltrials.gov
Last update: April 24, 2025
On this page, you'll find a regularly updated list of not yet recruiting clinical trials focused on novel therapies — including study details, sponsors, estimated enrolment, locations and the number of study centres.
A downloadable searchable Excel file to filter by sponsor, compound, or trial phase
(Updated regularly; the current file is from April 2025)
For an overview of currently recruiting clinical trials in CML, click here.
Disclaimer
The information presented in this overview has been sourced from www.clinicaltrials.gov and reflects data available as of April 24, 2025. While the iCMLf strives to ensure accuracy, it is not responsible for any errors, updates, or changes that may have occurred since that date. This list is provided solely for informational purposes and is organized alphabetically by investigational compound. The iCMLf does not endorse or recommend any specific clinical trial, investigational drug, or sponsor mentioned. For the most current and comprehensive details - including eligibility criteria, study locations, and contact information - please consult the official listing on clinicaltrials.gov.
| Compound | Brief description of the study | Sponsor | Status | Location / Centres |
| Asciminib |
PEARL Study: PotEntial of Asciminib in the eaRly Treatment of CML (PEARL)
|
Gruppo Italiano Malattie EMatologiche dell'Adulto Contact details: p.fazi@gimema.it |
Not yet recruiting |
Italy: Exact site information not available |
| Asciminib |
A Study of Treatment-free Remission in Chronic Phase Chronic Myeloid Leukemia |
Korean Society of Hematology Contact details: ksh.cml.wp@gmail.com |
Not yet recruiting Estimated enrolment: 69 participants |
Rep. of Korea: Exact site information not available |
| Asciminib | RWE, NIS, Prospective Study for the Effectiveness, Tolerability and Adherence of Asciminib in Saudi Arabia. (ASC4REAL) (ASC4REAL) (Clinicaltrials.gov Identifier: NCT06684964) This non-interventional study aims to evaluate the real-world effectiveness, tolerability, adherence, healthcare resource utilization, and patient-reported outcomes of asciminib in Ph+ CML-CP patients previously treated with at least two TKIs in Saudi Arabia. More information |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Not yet recruiting Estimated enrolment: 40 patients |
Saudi Arabia: Exact site information not available |
| HS-10382 |
Study of HS-10382 Combination in Patients With Chronic Myeloid Leukemia (CML) |
Jiangsu Hansoh Pharmaceutical Co., Ltd. Contact details: dr_huyu@126.com |
Not yet recruiting Estimated enrolment: 100 participants |
China: Exact site information not available |
| Olverembatinib (HQP1351) |
Phase II Study Assessing the Efficacy and Toxicity of Olverembatinib Monotherapy in Patients With Newly Diagnosed Chronic Myeloid Leukemia in |
MD Anderson Cancer Center Contact details: fhaddad@mdanderson.org |
Not yet recruiting Estimated enrolment: 50 patients |
USA: 1 |
| Olverembatinib (HQP1351) |
The Efficacy and Safety of Third-generation TKIs Combined With Azacitidine and Bcl-2 Inhibitor in Patients With CML-BP |
Peking University People's Hospital Contact details: jiangqian@medmail.com.cn |
Not yet recruiting Estimated enrolment: 30 participants |
China: 1 |
| Olverembatinib (HQP1351) |
PEDIATRIC CML STUDY Olverembatinib Combined With Venetoclax and Azacitidine in Blast Phase Ph Chromosome-positive CML |
Institute of Hematology & Blood Diseases Hospital, China Contact details: weihui@ihcams.ac.cn |
Not yet recruiting Estimated enrolment: 29 patients |
China: Exact site information not available |
|
TQB3911 |
A Clinical Trial to Evaluate the Safety and Tolerability of TQB3911 Tablets in Patients With BCR::ABL Fusion Gene Positive Leukemia |
Chia Tai Tianqing Pharmaceutical Group Nanjing Shunxin Pharmaceutical Co., Ltd. Contact details: jiangqian@medmail.com.cn |
Not yet recruiting Estimated enrolment: 120 participants |
China: 5 |
Overview of clinical trials in CML - Recruiting trials
New CML compounds in development
Source: www.clinicaltrials.gov
Last update: April 24, 2025
On this page, you'll find a regularly updated list of recruiting clinical trials focused on novel therapies — including study details, sponsors, estimated enrolment, locations and the number of study centres.
A downloadable searchable Excel file
spreadsheet
(28 KB)
to filter by sponsor, compound, or trial phase
(Updated regularly; the current file is from April 2025)
For an overview of clinical trials in CML not yet recruiting, click here.
Disclaimer
The information presented in this overview has been sourced from www.clinicaltrials.gov and reflects data available as of April 24, 2025. While the iCMLf strives to ensure accuracy, it is not responsible for any errors, updates, or changes that may have occurred since that date. This list is provided solely for informational purposes and is organized alphabetically by investigational compound. The iCMLf does not endorse or recommend any specific clinical trial, investigational drug, or sponsor mentioned. For the most current and comprehensive details - including eligibility criteria, study locations, and contact information - please consult the official listing on clinicaltrials.gov.
| Compound | Brief description of the study | Sponsor/ Contact details |
Status | Location / Centres |
| Asciminib |
PEDIATRIC CML STUDY: |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Recruiting Estimated enrolment: 34 participants |
USA: 8 |
| Asciminib |
Asciminib Roll-over Study This open-label, global roll-over study aims to assess the long-term safety of asciminib and provide continued treatment for patients who have completed a Novartis-sponsored asciminib study and are deemed to benefit from ongoing therapy. |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Recruiting Estimated enrolment: 347 participants |
USA: 4 |
| Asciminib |
Clinical Study of Asciminib in Previously Treated Indian Patients With Ph+ CML-CP Without T315I Mutation and in Patients With Ph+ CML-CP With T315I Mutation (ASC4INDIA) This Phase IV study aims to confirm the safety and efficacy of asciminib in Indian patients with Ph+ CML-CP, with and without the T315I mutation, following its approval by the Drugs Controller General of India. More information |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Recruiting Estimated enrolment: 85 participants |
India: 2 |
| Asciminib | Open-label Study of Asciminib for CML-CP or CML-AP Patients With T315I Mutation Who Are Resistant, Intolerant or Ineligible to Ponatinib. (ASC4TARGET) (Clinicaltrial.gov Identifier: NCT06514534) This Phase II study aims to evaluate the efficacy and safety of asciminib in managing CML-CP and CML-AP in patients with the T315I mutation, addressing a critical unmet need by exploring its potential as a new therapeutic option for those with limited treatment choices. More information |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Recruiting Estimated enrolment: 20 participants |
France: 1 |
| Asciminib |
Phase II Study Assessing Efficacy and Safety of Asciminib in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (Clinicaltrials.gov Identifier: NCT06236724) The study aims to evaluate the effectiveness and safety of asciminib in controlling chronic myeloid leukemia (CML) by assessing molecular and cytogenetic responses, treatment-free remission, survival outcomes, and adverse events over time. More information |
MD Anderson Cancer Center Contact details: ejabbour@mdanderson.org |
Recruiting |
USA: 1 |
| Asciminib |
Asciminib as Initial Therapy for Patients With Chronic Myeloid Leukemia in Chronic Phase (ALERTCML) (Clinicaltrials.gov Identifier: NCT05143840) |
Augusta University Contact details: |
Recruiting Estimated enrolment: 100 participants |
USA: 6 |
| Asciminib |
Asciminib Used in Consolidation With Imatinib vs. Imatinib to Achieve TFR in CP-CML |
Jewish General Hospital Contact details: clambert@jgh.mcgill.ca |
Recruiting Estimated enrolment: 164 participants |
Canada: 4 |
| Asciminib |
Treatment Free Remission After Combination Therapy With Asciminib (ABL001) Plus Tyrosine Kinase Inhibitors (TKI) in Chronic Phase Chronic Myeloid Leukemia (CP-CML) Patients Who Relapsed After a Prior Attempt at TKI Discontinuation
|
Medical College of Wisconsin Contact details: cccto@mcw.edu |
Recruiting Estimated enrolment: 51 participants |
USA: 4 |
| Asciminib |
Phase II Study of Asciminib for Second-line Treatment of Chronic Phase Chronic Myeloid Leukemia |
MD Anderson Cancer Center Contact details: gcissa@mdanderson.org |
Recruiting |
USA: 1 |
| Asciminib |
Asciminib Prospective Non-Interventional Study as 3rd Line Therapy or More to Treat Adult Patients With CML- CP in Real World Setting in France (ASSURE-3) |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Recruiting Estimated enrolment: 168 participants |
France: 38 |
| Asciminib |
Asciminib RMP Study |
Novartis Pharmaceuticals Contact details: novartis.email@novartis.com |
Recruiting Estimated enrolment: 100 participants |
Rep. of Korea: 14 |
| Asciminib/ Blinatumomab |
ABL001 + Dasatinib + Prednisone + Blinatumomab in BCR-ABL+ B-ALL or CML This study aims to evaluate the safety and efficacy of ABL001 in combination with dasatinib and prednisone as a potential treatment for BCR-ABL+ B-cell acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia in lymphoid blast crisis. |
Dana-Farber Cancer Institute Contact details: Marlise_Luskin@DFCI. HARVARD.edu |
Recruiting Estimated enrolment: 40 participants |
USA: 4 |
| ELVN-001 |
ELVN-001 for the Treatment of Chronic Myeloid Leukemia With and Without T315I Mutation in Japanese Participants (CML) |
Enliven Therapeutics Contact details: elvn-001-105@enliventherapeutics. com |
Recruiting Estimated enrolment: 21 participants Site recruitment closed. |
Japan: 4 |
| ELVN-001 |
A Phase 1a/1b Study of ELVN-001 for the Treatment Chronic Myeloid Leukemia (CML) |
Enliven Therapeutics Contact details: ELVN-001-101@enliventherapeutics. com |
Recruiting Estimated enrolment: 200 participants Site recruitment closed. |
USA: 3 |
| HS-10382 |
A Study of HS-10382 in Patients With Chronic Myeloid Leukemia
This study aims to evaluate the safety, tolerability, pharmacokinetics, and anti-CML activity of HS-10382, an oral allosteric BCR-ABL1 inhibitor, in patients with chronic myeloid leukemia (CML). More information |
Jiangsu Hansoh Pharmaceutical Co., Ltd. Contact details: dr_huyu@126.com |
Recruiting Estimated enrolment: 108 participants |
China: 1 |
| Olverembatinib (HQP1351) |
Study of Olverembatinib (HQP1351) in Patients With CP-CML (POLARIS-2) |
Ascentage Pharma Group Inc. Contact details: ben.little@ascentage.com |
Recruiting Estimated enrolment: 285 participants |
USA: 1 |
| Olverembatinib (HQP1351) |
Study of HQP1351 in Subjects With Refractory CML and Ph+ ALL |
Ascentage Pharma Group Inc. Contact details: Bill.Garrett@ascentage.com |
Recruiting Estimated enrolment: 62 participants |
USA: 8 |
| Olverembatinib (HQP1351) |
A Phase I Study of Decitabine, Lisaftoclax, and Olverembatinib in Patients With Advanced Chronic Myeloid Leukemia and Philadelphia Chromosome-Positive Acute Myeloid Leukemia |
MD Anderson Cancer Center Contact details: nshort@mdanderson.org |
Recruiting Estimated enrolment: 30 participants |
USA: 1 |
| Olverembatinib (HQP1351) |
Treatment With Olverembatinib in CML-CP Patients Who Failed to at Least Two Previously Administered Second-generation TKIs This study aims to evaluate the efficacy and safety of olverembatinib in chronic-phase CML patients who are resistant or intolerant to at least two second-generation TKIs, with efficacy measured by major molecular response (MMR) at 12 months. More information |
Shenzhen Second People's Hospital Contact details: duxingz@medmail.com.cn |
Recruiting Estimated enrolment: 40 participants |
China: 1 |
| Radotinib |
Efficacy and Safety of Dose Redution of Radotinib as a First Line Treament in Ph+ CML |
Il-Yang Pharm. Co., Ltd. Contact details: nykim@ilyang.co.kr |
Recruiting Estimated enrolment: 168 participants |
Rep. of Korea: 2 |
| Radotinib |
A Phase 3 Study for the Efficacy and Safety of Radotinib in CP-CML Patients With Failure or Intolerance to Previous TKIs |
Il-Yang Pharm. Co., Ltd. Contact details: nykim@ilyang.co.kr |
Recruiting Estimated enrolment: 173 participants |
Rep. of Korea: 1 |
| TERN-701 |
CARDINAL- A Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of TERN-701 in Participants With Chronic Myeloid Leukemia This study aims to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of TERN-701, a selective allosteric BCR-ABL1 inhibitor, in previously treated CP-CML patients through dose escalation and expansion phases. More information |
Terns, Inc. Contact details: cardinal@ternspharma.com |
Recruiting Estimated enrolment: 100 participants Still open for site recruitment |
USA: 9 |
| TGRX-678 |
TGRX-678 US Phase I for Subjects with Refractory or Advanced Chronic Myelogenous Leukemia |
Shenzhen TargetRx, Inc. Contact details: xinyi.zhu@tjrbiosciences. com |
Recruiting Estimated enrolment: 90 participants |
USA: 2 |
| TGRX-678 |
TGRX-678 Chinese Phase II in Chronic Myelogenous Leukemia (CML) Patients |
Shenzhen TargetRx, Inc. Contact details: yingkun.lv@tjrbiosciences. com |
Recruiting Estimated enrolment: 40 participants |
China: 1 |
CML Publications 2024
CML paper summary: The e13a3 (b2a3) and e14a3 (b3a3) BCR::ABL1 isoforms are resistant to asciminib (Leske IB and Hantschel O. Leukemia, Sept 2024)
CML paper summary - January 2025
The e13a3 (b2a3) and e14a3 (b3a3) BCR::ABL1 isoforms are resistant to asciminib
Leske IB and Hantschel O. Leukemia, September 2024
Leukemia 2024 Sep;38(9):2041-2045: https://www.nature.com/articles/s41375-024-02314-7
Introduction:
Asciminib, a first-in-class allosteric BCR::ABL1 inhibitor, has shown significant efficacy in patients with Philadelphia chromosome-positive chronic myeloid leukemia (CML) who have been previously treated with other tyrosine kinase inhibitors (TKIs). This study by Leske and Hantschel investigates the potential resistance of specific BCR::ABL1 isoforms to asciminib, particularly focusing on the e13a3 (b2a3) and e14a3 (b3a3) isoforms. These isoforms, which lack exon 2 of ABL1 and are present in less than 1% of CML patients, hypothesised to be resistant to asciminib due to the absence of a functional SH3 domain crucial for the drug's mechanism of action.
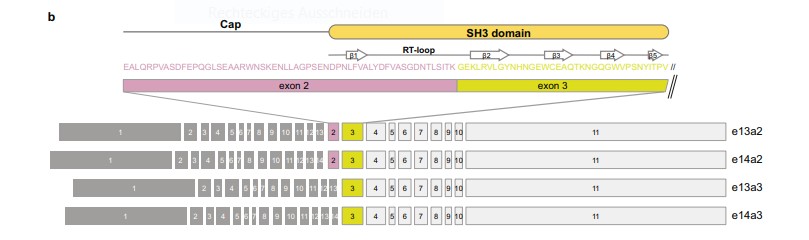
Fig 1: Overview of BCR::ABL1 exon organisation of the common e13a2, e14a2 transcript variants, as well as e13a3 and e14a3 that lack ABL1 exon 2.
Study design:
- Objective: To evaluate the resistance of BCR::ABL1 e13a3 and e14a3 isoforms to asciminib treatment.
- Methodology: The study involved generating cDNAs for the e13a3 and e14a3 isoforms and testing their response to asciminib in BaF3 cells. The effect of asciminib on BCR::ABL1-dependent cell proliferation and survival was measured using a range of asciminib concentrations.
- Comparison: The results for e13a3 and e14a3 were compared to the more common e14a2 isoform, which is known to be sensitive to asciminib.
Study results:
- Asciminib sensitivity:
- The e13a3 and e14a3 isoforms demonstrated significant resistance (~10,000-fold) to asciminib, with no inhibition observed at concentrations below 50 µM. In contrast, the e14a2 isoform showed a half-maximal growth inhibition (GC50) of approximately 1 nM.
- Both e13a3 and e14a3 isoforms were highly sensitive to dasatinib, an ATP-competitive inhibitor.
- Mechanism of resistance:
- The resistance observed for the e13a3 and e14a3 isoforms was attributed to the absence of a functional SH3 domain, which is required for asciminib's allosteric inhibition of BCR::ABL1. Structural modelling and binding studies confirmed that the lack of exon 2 disrupts the SH3 domain, preventing the necessary conformational changes for asciminib-mediated inhibition.
- Immunoblotting revealed that STAT5A/B phosphorylation, critical for leukemogenesis, remained unaffected by asciminib in e13a3 and e14a3 cells.
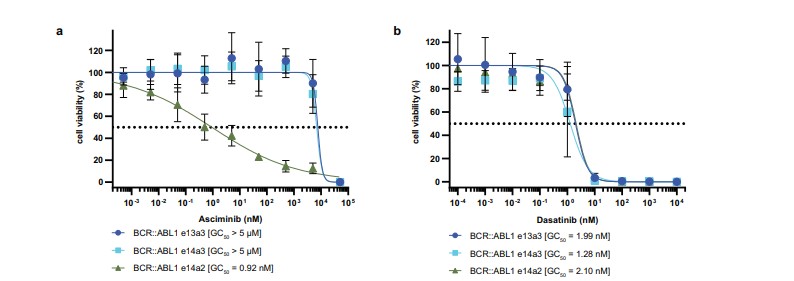
Fig 2: BCR::ABL1 e14a2 and e14a3 are asciminib resistant, but sensitive to dasatinib. Normalised viability of Ba/F3 cells expressing BCR::ABL1 e14a2, e14a3 and e13a3 fusion transcripts was measured in the presence of the indicated concentrations of asciminib (a)
or dasatinib (b) after 48 h.
Conclusion
This study provides evidence of a primary resistance mechanism to asciminib in CML patients with the e13a3 and e14a3 BCR::ABL1 isoforms. These findings are crucial for personalising treatment strategies in CML and suggest that ATP-competitive BCR::ABL1 inhibitors, such as dasatinib, should be preferred for patients with these isoforms. Genetic screening for BCR::ABL1 isoforms is crucial before initiating therapy to ensure optimal treatment selection for CML patients.
iCMLf Forum 2024 - Meeting Summary
Topic: Advancing Treatment-Free Remission (TFR) in CML Worldwide
Date: December 6, 2024 | Location: San Diego (USA)
Meeting chair: Dr Katia Pagnano, HEMOCENTRO / UNICAMP – Brazil)
The 2024 iCMLf Forum focused on advancing Treatment-Free Remission (TFR) for CML globally. Expert speakers shared progress in research, diagnostics, and strategies for enhancing TFR access, particularly in low- and middle-income countries (LMICs).
 Session 1: The iCMLf – Planning for the future
Session 1: The iCMLf – Planning for the future
Speaker: Nicola Evans, Chief Executive, iCMLf
Nicola Evans provided an overview of iCMLf’s mission, vision and future direction, emphasising the continued support for equal access to treatment, diagnostics, and care for all CML patients.
The iCMLf leadership is finalising the strategies and projects for the next 10 years.
This will focus on:
- Expanding CML education globally with a dedicated focus on LMICs
- Increasing access to diagnostics
- Providing overarching global patient support and information
- Continuing to research optimal treatment pathways
More details to follow in early 2025…
Session 2: Understanding the Optimal Pathway to Successful TFR
 Presentation: Biological Barriers to Cure
Presentation: Biological Barriers to Cure
Speaker: Professor Brian J. Druker, OHSU Knight Cancer Institute, USA
Professor Druker discussed intrinsic and extrinsic barriers preventing TFR and proposed research strategies to overcome them.
Key Points:
- Barriers to achieving TFR:
- Cell-intrinsic factors: Kinase-independent survival pathways, epigenetic reprogramming, and metabolic adaptations allow residual leukemia cells to persist.
- Cell-extrinsic factors: Immune system interactions, inflammatory responses, and the bone marrow niche play critical roles in maintaining minimal residual disease (MRD).
- Long-term patient management:
- Achieving deep molecular response remains a priority, with current therapies enabling TFR in only 10-20% of patients.
- Shared decision-making is vital for managing treatment cessation, balancing benefits (e.g., reduced toxicity) with potential relapse.
- Strategic goals for future therapies:
- Targeting residual leukemic stem cells through safe, precise therapies.
- Utilising global sample repositories to identify novel therapeutic targets and biomarkers for TFR success
Presentation: Innovative Biomarkers for TFR
Professor Ong presented data on biomarkers predicting TFR success and relapse, using single-cell technologies to identify stem cell differences.
Key Points:
- Role of leukemic stem cells (LSCs):
- Pre-treatment biology at the LSC level determines TFR outcomes, with specific biomarkers predicting relapse or sustained remission.
- Patients with high LSC burden or certain genetic signatures exhibit higher risk of relapse post-TKI cessation.
- Predictive biomarkers:
- Rapid BCR::ABL1 decline at three months is linked to long-term TFR success, suggesting favourable pre-existing stem cell biology.
- Identification of cell surface protein biomarkers (e.g., in CD34+ cells) at diagnosis may enable patient stratification for TFR attempts.
- Single-cell technologies:
- Leveraging single-cell RNA sequencing to map stem cell states, highlighting proliferative pathways (e.g., IL-2/STAT5 signalling) in relapsed patients.
- Epigenetic gene sets in relapsed LSCs provide potential therapeutic targets for future interventions.
Session 3: Successful TFR Globally
 Presentation: TFR in Low-and-Middle-Income Countries
Presentation: TFR in Low-and-Middle-Income Countries
Professor Akhil Ranjon Biswas, Dhaka Medical College, Bangladesh (presented by Dr Shanmuganathan)
Insights on TFR in LMICs, including how this can be achieved, patient perceptions and the challenges of limited access to TKIs, testing, and monitoring infrastructure.
Key Points:
- Access barriers in LMICs:
- Limited availability of TKIs due to lack of insurance and reliance on out-of-pocket expenses.
- Variable access to molecular testing with concerns over sensitivity, reliability, and high costs.
- Practical monitoring solutions:
- Use of FISH cytogenetics and qualitative PCR as cost-effective alternatives to quantitative BCR-ABL testing.
- Reducing test frequency while maintaining safety, e.g., bimonthly testing in the first six months.
- Improving patient compliance:
- Health literacy programs to educate patients on the importance of adherence and timely molecular monitoring.
- Strengthening government support for free TKIs and subsidised diagnostics.
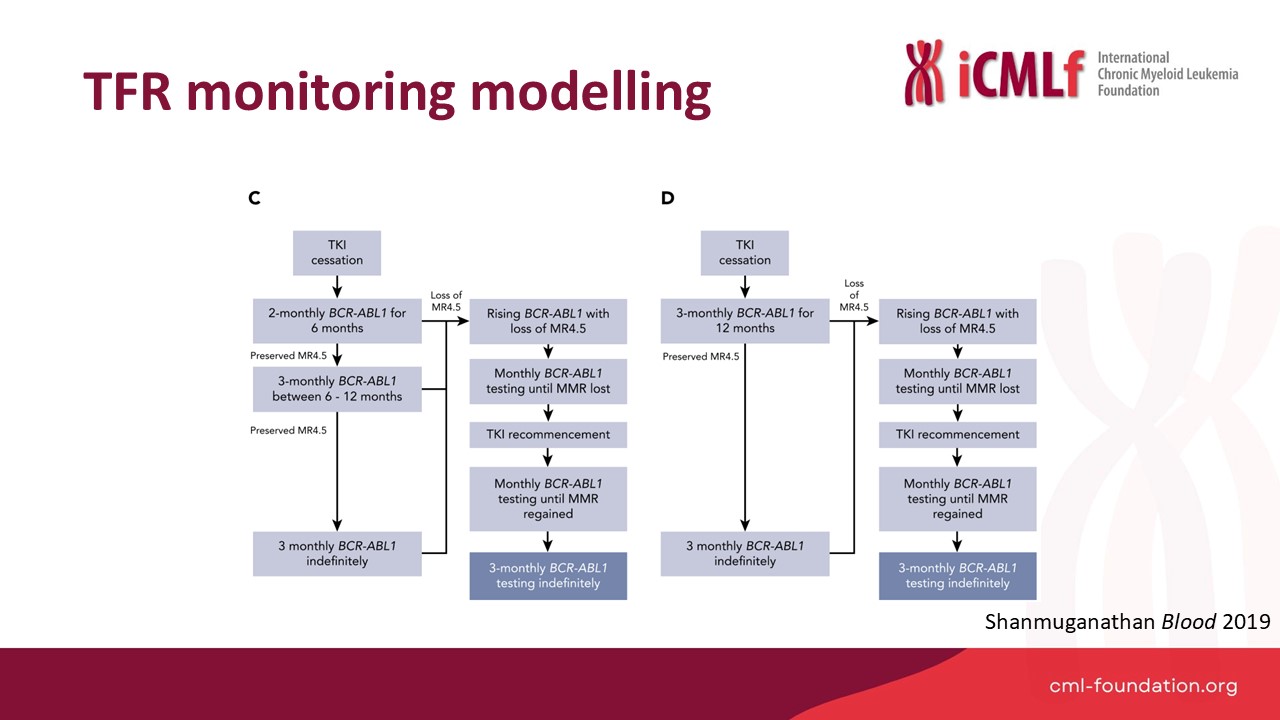 Presentation: Optimal TFR Monitoring
Presentation: Optimal TFR Monitoring
Speaker: Dr Naranie Shanmuganathan, Royal Adelaide Hospital, Australia
Dr Shanmunagathan presented optimised TFR monitoring strategies and practical approaches to improve global accessibility.
Key Points:
- Optimised monitoring protocols:
- Modelling reduced testing regimes (e.g., monthly for six months, then bi-monthly) showed significant cost reductions while maintaining safety.
- Real-world implementation of updated NCCN and ELN guidelines would reduce testing burden.
- Global disparities:
- Successful TFR programs rely on reliable, timely, and affordable BCR::ABL1 testing infrastructure.
- Regional and national efforts must address gaps in testing capabilities for widespread adoption of TFR strategies.
 Presentation: Expanding Diagnostics for CML
Presentation: Expanding Diagnostics for CML
Speaker: Professor Jerry Radich, Fred Hutchinson Cancer Center, USA
Professor Radich highlighted novel diagnostic solutions, including portable PCR devices, lateral flow assays, and nanopore sequencing for LMICs.
Key Points:
- InnovativeV diagnostic tools:
- Development of electricity-free PCR and lateral flow assays as affordable, portable options for LMICs.
- Digital PCR offers superior sensitivity compared to conventional RT-PCR for detecting MRD and predicting relapse.
- Advances in sequencing technologies:
- Portable nanopore sequencing provides real-time results, enabling genetic analysis without reliance on centralised facilities.
- Integration of sequencing data into mobile applications allows for rapid diagnostics and results sharing in remote areas.
- Future directions:
- Use of dried blood spot technology for multiplexed testing, including resistance mutations and whole-genome panels.
- Expanding programs like “Spot on CML” to support large-scale diagnostics globally.
Panel Discussion
Moderator: Giora Sharf, CML Advocates Network
Panellists shared insights on the challenges and future of TFR, emphasising practical, affordable solutations for LMICs.
Key Topics:
- Balancing monitoring rigor and accessibility:
- Flexible protocols (e.g., reduced testing frequency) are necessary for resource-limited settings without compromising safety.
- Long-term monitoring beyond five years:
- Shared decision-making with patients is critical, with proposals for six-monthly or annual testing based on individual risk.
- Practical TFR implementation in LMICs:
- Strategies to increase government-funded TKIs and diagnostics, alongside education programs to improve compliance and patient outcomes.
Conclusion
The 2024 iCMLf Forum delivered actionable insights to advance TFR worldwide. Experts emphasised collaboration, innovation, and equity in addressing barriers to care, particularly in LMICs, with a shared vision of achieving a cure for CML.










