CML paper summary - October 2024
Point-of-care BCR::ABL1 transcript monitoring using capillary dried blood in CML patients
Sala-Torra O et al. Leukemia, June 2024
Leukemia (2024) 38:1822–1824 https://doi.org/10.1038/s41375-024-02285-9
Introduction:
This study explores a novel method for monitoring BCR::ABL1 transcripts in CML patients using a point-of-care dried capillary blood (DCB) collection system. With CML requiring lifelong treatment and frequent monitoring, the method aims to address the challenges faced by patients in remote locations or with limited access to healthcare facilities.
Study design:
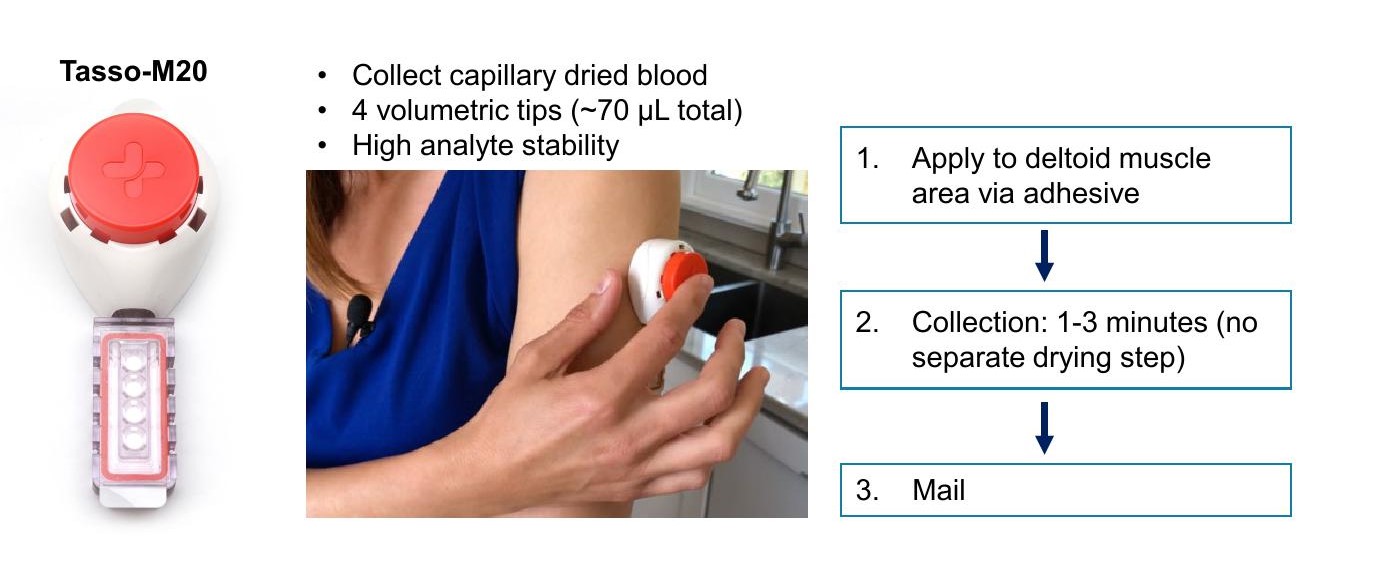 The researchers developed and evaluated a capillary dried blood collection method using the Tasso-M20 device.
The researchers developed and evaluated a capillary dried blood collection method using the Tasso-M20 device.- Twenty patients enrolled at Fred Hutchinson Cancer Center were monitored using DCB samples, which were compared with standard venous blood monitoring via quantitative reverse-transcription polymerase chain reaction (RT-PCR).
- The samples were tested for BCR::ABL1 transcripts using the Xpert® BCR-ABL Ultra assay (Cepheid).
Study endpoints:
- The primary goal was to assess the correlation between BCR::ABL1 transcript levels in DCB and standard venous blood samples.
- Key outcomes included major molecular response (MMR) rates and overall sensitivity of the DCB method compared to venous blood.
Study results:
- DCB collection using the Tasso-M20 device was well-tolerated, with patients reporting minimal pain (mean pain score <1.5).
- Strong correlations (r=0.96) between BCR::ABL1 levels in DCB and venous samples were observed.
- Fifteen patients achieved MMR by DCB measurements, of which 12 were concordant with venous blood results.
- Patients with BCR::ABL1 transcripts <0.09% in venous blood were not reliably detected by the DCB method.
Discussion:
The study demonstrates the feasibility of patient-directed DCB collection for CML monitoring, providing a low-cost, stable, and convenient alternative to traditional venous blood testing. While the method is effective for detecting MMR and loss of response, its sensitivity for deeper molecular responses may be limited due to the smaller blood volume.
Future improvements, including larger sample collections, could enhance detection sensitivity.
Conclusion:
Capillary dried blood sampling offers a promising point-of-care tool for CML monitoring, especially in resource-limited settings. However, further developments are required to expand its use in treatment-free remission assessments.










