 Twenty-five years ago, the landscape of chronic myeloid leukemia (CML) treatment changed forever with the introduction of tyrosine kinase inhibitors (TKIs). Before this breakthrough, CML was a life-threatening diagnosis, with limited treatment options such as interferon therapy or bone marrow transplantation. Today, thanks to TKIs, CML has transformed into a manageable condition, allowing patients to live full and productive lives.
Twenty-five years ago, the landscape of chronic myeloid leukemia (CML) treatment changed forever with the introduction of tyrosine kinase inhibitors (TKIs). Before this breakthrough, CML was a life-threatening diagnosis, with limited treatment options such as interferon therapy or bone marrow transplantation. Today, thanks to TKIs, CML has transformed into a manageable condition, allowing patients to live full and productive lives.
The Birth of Targeted Therapy
The story began in 1998 with the first clinical trials of imatinib (Gleevec/Glivec), a drug specifically designed to target the BCR-ABL fusion protein—the genetic driver of CML. This revolutionary approach, pioneered by Dr. Brian Druker and colleagues demonstrated unprecedented efficacy in the Phase I and II clinical trials beginning in 2000, leading to FDA approval of imatinib in 2001.
Imatinib’s success ushered in a new era of targeted therapy, inspiring the development of second- and third-generation TKIs such as dasatinib, nilotinib, bosutinib, ponatinib and now the STAMP inhibitor, asciminib. These drugs provided options for patients with resistance or intolerance to first/second line treatment, enhancing treatment decisions to achieve effective therapy.
Transforming Lives
The impact of TKIs on patient outcomes has been nothing short of extraordinary. Before their introduction the five-year survival rate for CML was around 30%. Today, more than 90% of patients achieve long-term remission, with many leading near-normal lives. Moreover, molecular monitoring advancements have allowed for precise treatment adjustments, and treatment-free remission has become a reality for some patients who achieve deep, sustained responses.
Yet, the work is far from done. Today, we celebrate this remarkable achievement while recognising the challenges that remain. Access to TKIs is still limited in many parts of the world, and we must continue to fight for equitable care for all.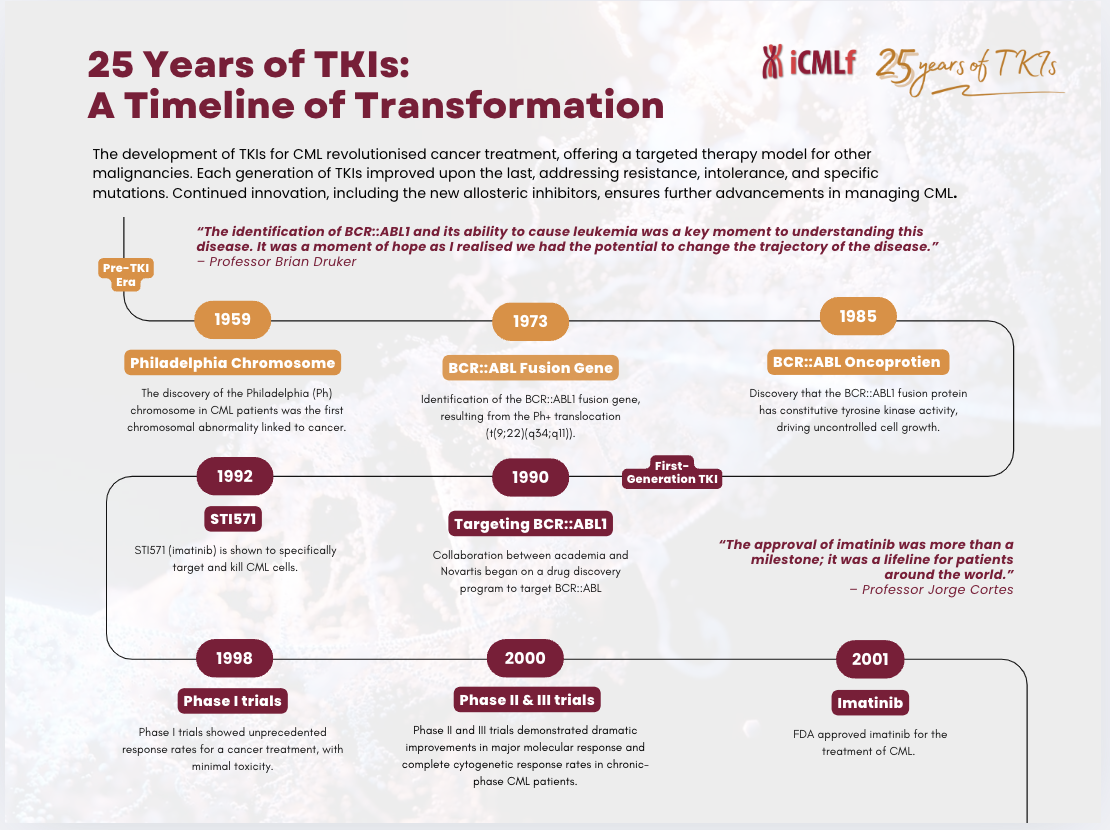
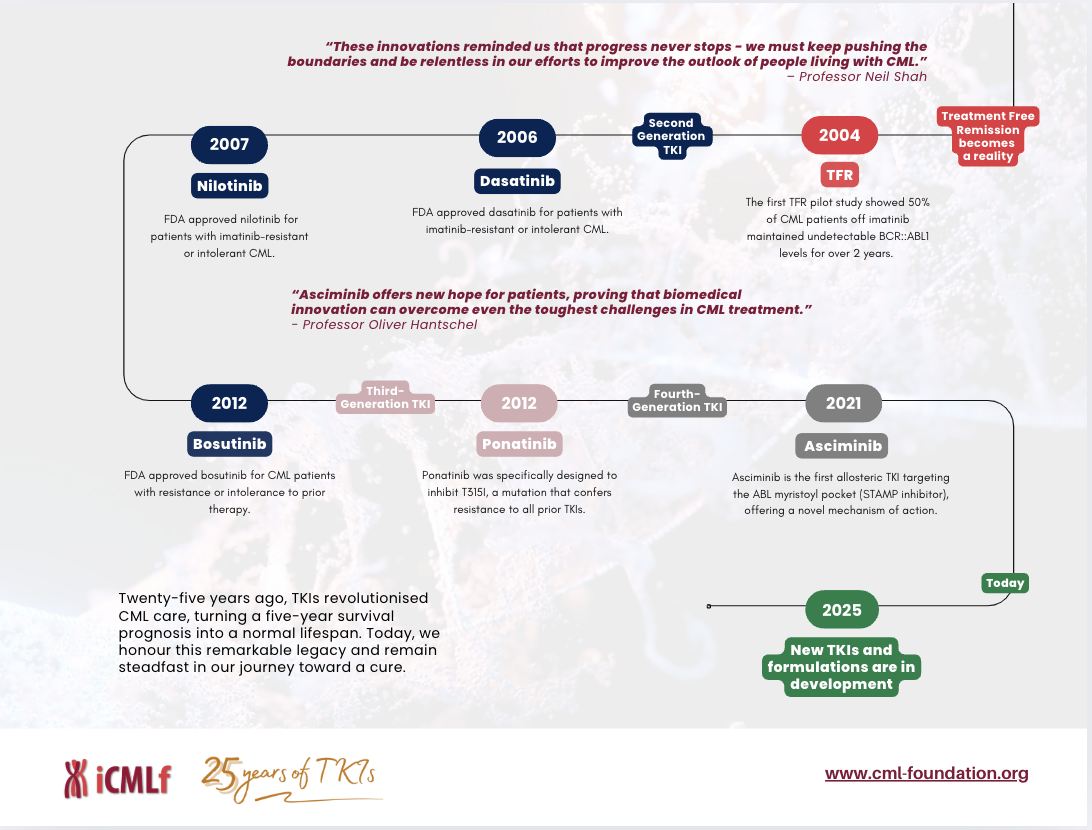
Click here to download a pdf of our visual -25 Years of TKIs: A Timeline of Progress
Looking Ahead
As we celebrate 25 years of TKIs, we also look to the future. Ongoing research aims to refine treatment strategies, reduce side effects, and explore potential curative approaches. With continued innovation and global access initiatives, the goal is to ensure that every person diagnosed with CML can benefit from these life-changing therapies.
The journey from a once-fatal disease to a manageable condition is a testament to the power of scientific discovery, perseverance, and patient advocacy. As we mark this milestone, we honor the researchers, clinicians, and-most importantly-patients who have shaped the incredible success story of TKIs in CML treatment.
TKIs are central to the mission of the iCMLf - guiding our research, informing our educational initiatives, and inspiring our advocacy for patients everywhere.
We invite you to join us in reflecting on the progress we’ve made and the future we envision. Throughout 2025, we’ll highlight the milestones of this incredible journey, share personal stories from those whose lives have been transformed, and explore the exciting innovations on the horizon.
Together, we honor the past, celebrate the present, and strive for a future where every person with CML has access to life-saving treatments - and where a cure is within reach.
Stay tuned for stories, events, and opportunities to get involved in this year of celebration.
Join us in celebrating 25 years of TKIs - a legacy of hope and progress. Download the timeline here -25 Years of TKIs: A Timeline of Progress
Imatinib TM Glivec/Geevec manufactured by Novartis Pharmaceuticals Bositinib ™ Bosulif manufactured by Pfizer Ponatinib ™ Iclusig manufactured by ARIAD pharmacuticals, currently Takeda and Incyte Asciminib ™ Scemblix manufactured by Novartis Pharmaceuticals










