We're bringing you daily updates from the CML sessions at EHA2024. Check back each day for updates on the day's sessions.
We're bringing you daily updates from the CML sessions at EHA2024. Check back each day for updates on the day's sessions.
Those registered for EHA can view the session on demand.
Day 4: Sunday 16th June 2024
Oral Session: Optimisation and Innovation in CML Treatment
During the oral session ‘Optimisation and Innovation in CML treatment’, speakers provided an update on latest clinical trials on CML.
Our key takeaways from the presentations:
1) Ponatinib and 5-Azacytidine for the treatment of chronic myelogenous leukemia in myeloid blast crisis. Results of the PONAZA trial
Speaker: Philippe Rousselot, University de Versaille St-Quentin-en-Yveline (Paris, France)
“High response rates without excessive toxicity, regardless of age, highlight the success of combining 5-azacytidine and ponatinib in treating CML." -Philippe Rousselot
Key points:
- High response rate with 5-azacytidine and ponatinib: The combination is associated with a high response rate without excessive toxicity in patients with a median age of 60 years.
- Favourable comparison to intensive chemo and TKIs: Results are favourable compared to intensive chemotherapy combined with TKIs, despite the older age of the patients.
- Excellent outcomes with or without transplantation: All patients who underwent transplantation experienced excellent outcomes, while half of the patients who did not undergo transplantation also had excellent outcomes.
- Hematological response critical for survival: Hematological response was a determinant for survival, but the depth of molecular response was not.
2) SUSTRENIM Trial: Sustained deep molecular response and TFR rate in the long-term follow-up
Speaker: Massimo Breccia, Sapienza University (Rome, Italy)
“Further investigation is essential to fully grasp the benefits of switching to second-generation TKIs like nilotinib for patients who do not optimally respond to imatinib." – Massimo Breccia
Key Points:
- Overview of the SUSTRENIM trial: The SUSTRENIM trial is an International, prospective, randomised study with two arms. It aims to evaluate deep molecular response and TFR rates in newly diagnosed CP-CML patients assigned to either nilotinib or imatinib, with a switch to nilotinib if an optimal response is not achieved.
- Higher discontinuation eligibility with nilotinib: Long-term follow-up indicates a higher rate of therapy discontinuation eligibility in patients treated with nilotinib.
- Effectiveness of switching to nilotinib: Switching from imatinib to nilotinib, whether early or late due to non-optimal response, shows similar effectiveness in achieving deep molecular response.
- Comparison of TFR rates between nilotinib and imatinib: At 18 months, the TFR rate was higher in the nilotinib arm (74.4%) compared to the imatinib arm (44%), but the difference was not statistically significant.
Need for extended follow-up for second-generation TKIs: Further follow-up is necessary to fully understand the benefits of switching to second-generation TKIs, like nilotinib, for TFR in cases of non-optimal response.
3) Improved tolerability with dasatinib 5 days compared to 7 days per week in patients with chronic myeloid leukemia in chronic phase. Final results of the DASAHIT trial.
Speaker: Paul La Rosée, Schwarzwald-Baar Klinikum (Villingen-Schwenningen, Germany)
"Exploring intermittent dosing schedules for TKIs not only ensures safety for newly diagnosed patients but also opens avenues for enhancing quality of life outcomes, pending further study."
– Paul La Rosée
Key Points:
- Intermittent dosing trial findings: The first prospective, randomised multicentre trial shows non-inferiority in maintaining major molecular response (MMR) at 24 months with an intermittent dosing schedule, achieving a 29% cumulative weekly dose reduction.
- Caution in second-line patients: Non-inferiority of intermittent dosing may not apply to second-line patients, requiring careful consideration in daily practice.
- Reduction in pleural and pericardial effusions: Intermittent dosing significantly reduces adverse events such as pleural and pericardial effusions by 50%.
- Safety of intermittent dosing for front-line patients: Demonstrates a safe de-escalation strategy for newly diagnosed patients.
- Anticipated quality of life data: Awaiting results on the impact of intermittent dosing on patient quality of life.
4) Outcomes with ponatinib in patients with chronic-phase chronic myeloid leukemia and the T315I mutation: 4-year results from the OPTIC trial
Speaker: Jane Apperley, Imperial College London (London, United Kingdom)
" As we navigate individualised approaches, these results affirm ponatinib's efficacy in challenging cases of CML with T315I mutation." – Jane Apperley
Key points:
- 4-year post hoc analysis results: This post hoc analysis presents the 4-year follow-up results from the OPTIC trial for patients with the T315I mutation, supporting ponatinib’s long-term efficacy and manageable safety profile.
- Consistency with previous analyses: Despite smaller patient numbers in the mutation and dosing cohorts in the post hoc analysis, the results for CP-CML patients with the T315I mutation are consistent with previous OPTIC trial analyses.
- Long-term safety with response-based dosing: Response-based dosing with ponatinib showed long-term manageable safety, including a low exposure-adjusted rate of arterial occlusive events of approximately 2% in the 45 mg cohort.
- Association with long-term survival: Observed responses were linked to robust long-term survival in CP-CML patients resistant to second-generation BCR-ABL1 TKI therapy, with or without the T315I mutation; neither progression-free survival nor overall survival was reached in the 45 mg cohort for patients with the T315I mutation.
5) A phase 2, randomised trial of ruxolitinib in addition to BCR::ABL1 in CML patients with molecular evidence of disease (SWOG Trial S1712)
Speaker: Kendra Sweet, H. Lee Moffitt Cancer Center and Research Institute (Tampa, USA)
" As we explore this combination further, its impact on improving patient outcomes and expanding TFR eligibility warrants deeper investigation in larger phase 3 trials." – Kendra Sweet
Key points:
- Ruxolitinib addition to TKIs: Adding ruxolitinib to TKIs in CP-CML patients significantly increases rates of MR4.0 and MR4.5 at 12 months.
- More patients meet NCCN criteria: Significantly more patients treated with ruxolitinib meet the NCCN criteria for TKI cessation.
- Tolerability of the combination: The combination of ruxolitinib and TKIs is well tolerated.
- Ongoing research on patient benefits: Additional studies are ongoing to identify patients most likely to benefit from ruxolitinib.
- Need for larger phase 3 study: Investigating the combination of ruxolitinib and TKIs in a larger phase 3 study could determine if it leads to higher TFR rates.
Day 3: Saturday 15th June 2024
Scientific Working Group Session ELN-EHA-SWG for CML Highlights
At today’s ELN-EHA-SWG session for CML, the focus was on cutting-edge research into BCR-ABL1 inhibition, the role of BCL2 in myeloid malignancies, and the potential for leukemic stem cell inhibition. Here’s a summary of the key points and comments from each presentation:
1) Protac Inhibition of BCR-ABL1
Speaker: Michael Deininger (Milwaukee, WI, USA)
“The potential of PROTACs lies in their ability to specifically target and degrade the BCR-ABL protein, addressing both kinase-dependent and independent pathways.” - Michael Deininger
Key Points:
- Kinase-Independent Functions: Inhibiting BCR-ABL1 entirely is more potent than just blocking its kinase activity.
- PROTACs: These hetero-bifunctional molecules effectively degrade BCR-ABL1 by recruiting it to the ubiquitin-proteasome system.
- Clinical Trials: Advanced PROTACs for BCR-ABL1 are in clinical trials, showing promising results in targeting both wild-type and mutant forms.
- Key Findings: The novel PROTACs have demonstrated effective degradation of BCR-ABL1, including resistant mutants, pointing to a potential for improved therapeutic outcomes.
2) BCL2 Family in Myeloid Malignancies
Speaker: Caroline Heckman (Helsinki, Finland)
“Understanding the differential expression of BCL2 family members is crucial for developing targeted therapies.” - Caroline Heckman
Key Points:
- BCL2 Family Members: Different members play distinct roles in apoptosis regulation, impacting treatment outcomes.
- Venetoclax: Effective against immature AML subtypes but less so for more mature myeloid cells.
- Combination Therapies: Combining BCL2 inhibitors with other drugs, such as TKIs, shows promise in enhancing treatment efficacy.
- Clinical Implications: Targeting specific BCL2 family members could lead to more effective treatments for various myeloid malignancies.
3) Potential for Leukemic Stem Cell (LSC) Inhibition
Speaker: David Vetrie (Glasgow, United Kingdom)
“By using cytokines to reprogram LSCs, we can potentially improve the overall clinical outcome for patients.” - David Vetrie
Key Points:
- Single-Cell RNA-Seq: Revealed significant molecular diversity in LSCs from responders and non-responders.
- Quiescent LSCs: Differences in MYC and TNF-alpha/NF-kappa B signalling pathways were noted between responders and non-responders.
- Cytokine Exposure: Reprogramming LSCs with cytokines increased TNF-alpha signalling and improved TKI sensitivity in bulk tumors.
- Future Directions: Further research into cytokine-mediated reprogramming could lead to innovative treatments targeting LSCs and enhancing patient outcomes.
Day 2: Friday 14th June 2024
Haematology in Focus: CML Session
“We must join hands together. I am really proud to be part of the iCMLf, we’ve had really good interactions with them and their experts, and what we do will influence the world.”- Hemant Malhotra
At this morning’s session ‘Haematology in Focus: CML’, three insightful presentations provided valuable updates on alternative therapies, CML management in resource-limited settings, and patient monitoring. Here’s a summary of the key points and quotes from each speaker:
1) When Are Alternative Therapies Indicated?
Speaker: Andreas Hochhaus, Universitätsklinikum Jena (Jena, Germany)
“It’s crucial to consider the biological situation of each patient rather than rigidly following treatment lines.” - Andreas Hochhaus
Key Points:
- Indications for Changing Therapy: Lack of response, loss of response, grade 3 and 4 intolerance, and failure to achieve deep molecular response.
- Patient-Specific Treatment Goals: Treatment should be tailored based on TFR goals, mutations, intolerance profiles, and comorbidities.
- Lines of Therapy: The focus should be on the patient’s biological situation rather than strictly adhering to first, second, or third-line treatments.
- Molecular Milestones: It’s important to balance achieving major molecular response with quality of life and the risk of side effects.
2) Management of CML Patients in Countries with Limited Resources
Speaker: Hemant Malhotra, Mahatma Gandhi Medical College Hospital (Jaipur, India)
“Prevalence of CML is expected to increase hugely in my country and in the developing world and we really need to gear up to take care of these large number of patients. Hemant Malhotra
Key Points:
- Population context: CML is the most common adult leukemia in India and in next five years expecting over 150,000 patients with CML.
- Resource Constraints: Many patients in India lack medical insurance and must pay out-of-pocket, making expensive treatments impractical. Support programs like the Max Foundation’s GPAP provide crucial access to medications for many patients.
- Delayed Diagnosis: CML is often diagnosed later in low and middle-income countries, leading to larger loads and lower response rates.
- Tailored Guidelines: There’s a need for region-specific guidelines and consensus statements to address the unique challenges faced by patients in developing countries.
- Looking forward: TFR is even more important due to the younger population, and there must be a move to ensuring reliable, affordable BCR::ABL testing are critical steps.
3) Monitoring of CML Patients During and After Therapy
Speaker: Nicholas Cross, University of Southampton (Salisbury, United Kingdom)
“Reducing monitoring frequency once MMR is achieved and confirmed is a topic for discussion in future recommendations.” - Nicholas Cross
Key Points:
- Diagnostic Techniques: A wide range of techniques including RT-PCR, RNA sequencing, and NGS panels for precise diagnosis and monitoring.
- Molecular Monitoring: Essential for assessing treatment continuation and also eligibility for TFR. Digital PCR is a viable alternative to qPCR.
- Monitoring Frequency: Potential reductions to the recommended monitoring schedule are under discussion. Reducing monitoring frequency will depend on disease dynamics and patient specific factors
- Atypical Fusions: Special attention is needed for patients with atypical BCR-ABL fusions, who cannot be monitored on the international scale.
Education Session on CML: Updates in CML Management
At this morning’s Education Session ‘Updates in CML Management’, we learned about new targets outside of BCR-ABL, the role of quality of life as a CML treatment paradigm and new trends and latest discussions in CML therapy.
Our key takeaways from the presentations:
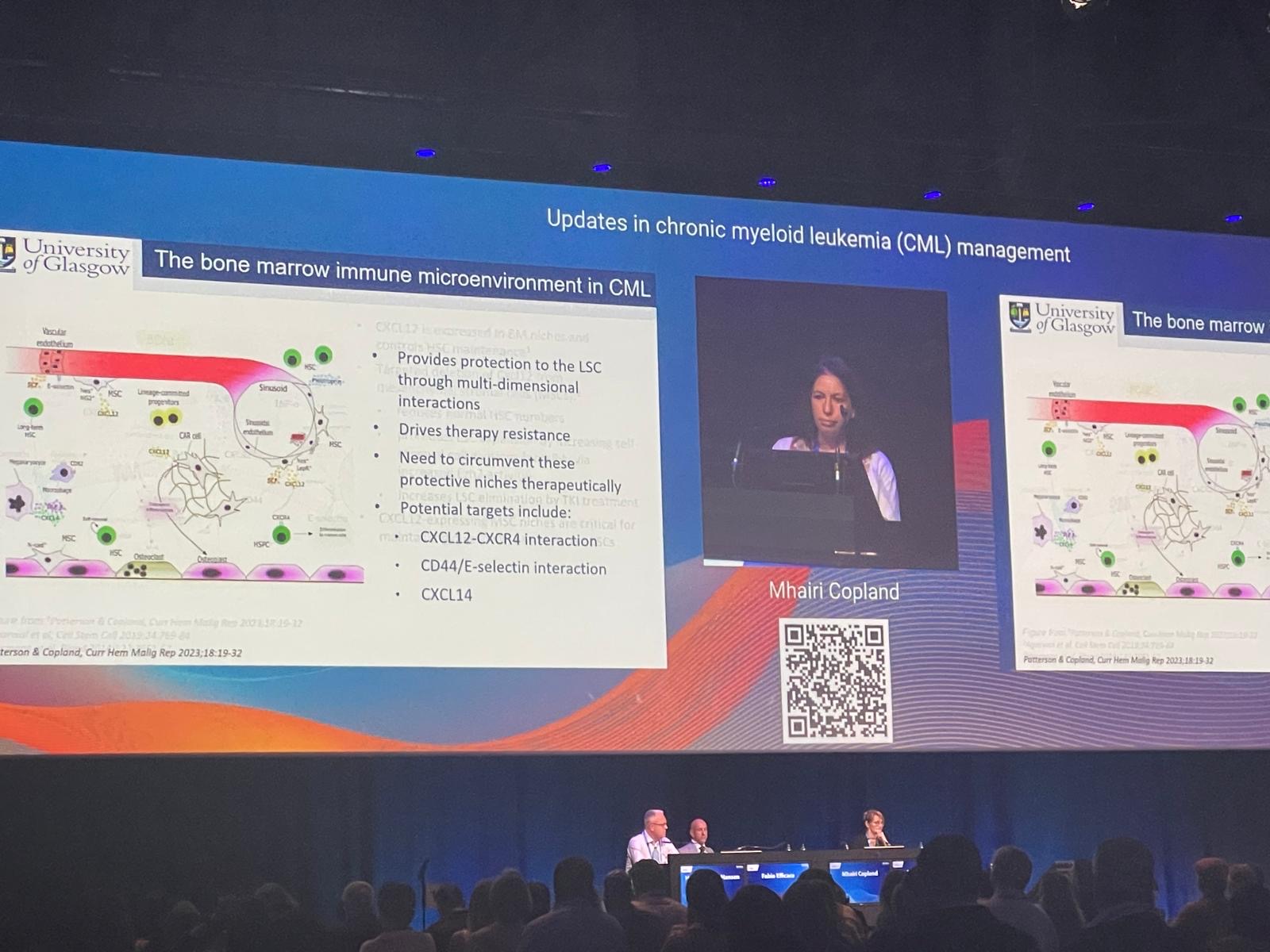
1) New targets outside of BCR-ABL
Speaker: Mhairi Copland, University of Glasgow (Glasgow, UK)
‘CML remains a model disease for understanding cancer biology and progression.”
- Mhairi Copland
Key points:
- Challenges in stem cell elimination: Despite the approval of several increasingly potent TKIs eliminating stem cells remains elusive
- Need for new therapeutic strategies: With the success of TFR in only a small proportion of patients, developing new therapeutic strategies aimed at cure remains a clinical need
- Potential new targets in bone marrow and microenvironment: Recent research emphasised the bone marrow niche and immune microenvironment interactions as promising targets for therapy
- Advancements in leukemic stem cell (LSC) metabolism targeting: Targeting LSC metabolism is a rapidly expanding field with the potential to repurpose existing widely used drugs
- Therapeutic challenges in blast phase: The blast phase of CML presents significant therapeutic challenges, but combining TKIs with strategies targeting the intrinsic apoptotic pathway and epigenetic regulators shows promise
2) New CML treatment paradigms: Improving quality of life in CML
Speaker: Fabio Efficace, GIMEMA (Rome, Italy) / Northwestern University (Chicago, USA)
“Quality of life is a crucial consideration for patients facing lifelong treatment. Understanding and measuring treatment impact on QoL is essential for informed decisions in managing the complexities of CML therapy. – Fabio Efficace
Key Points:
- Importance of QoL in clinical trials: Considering quality of life as a crucial endpoint in clinical trials helps to capture the impact of therapy from the patient's perspective, emphasising the importance of appropriate methodologies.
- Role of QoL monitoring in clinical practice: Routine practice that includes systematic monitoring of QoL and symptoms in CML patients can significantly enhance adherence to therapy, potentially improving clinical outcomes.
- Need for standardized monitoring approaches: There is a need to develop and implement standardised protocols for monitoring QoL and symptoms in routine CML practice.
- QoL benefits on treatment discontinuation: Discontinuing TKI treatment can lead to improvements in quality of life in various domains. However, the extent of these improvements may differ across age groups, with younger patients often seeing more significant benefits
3) Current trends and discussions in CML treatment
Speaker: Henrik Hjort-Hansen, Norwegian University of Science & Technology (Trondheim, Norway)
"The trend is moving towards personalised dosing and flexible treatment strategies to optimise outcomes across different age groups." – Henrik Hjort-Hansen
Key Points:
- Debate on Milestone Criteria: Ongoing debate surrounds the biological accuracy of current failure and milestone criteria in CML, particularly regarding patients exceeding specific BCR-ABL levels.
- Impact of Age on Treatment Outcomes: Variations in treatment outcomes based on age were underscored prompting discussions on adjusting milestones and criteria for different age cohorts.
- Dosing Flexibility: There is a need for dosing flexibility to manage side effects and enhance patient outcomes, reflecting a shift towards personalized dosing based on individual responses and tolerability.
- Accelerated Phase: The debate on including the accelerated phase in CML guidelines focuses on its distinct prognosis and varying treatment needs, prompting considerations for age-specific treatment milestones.
- Treatment-Free Remission: Exploring treatment discontinuation in CML focuses on achieving deep molecular responses, monitoring relapse kinetics, and refining dosing strategies to optimise long-term outcomes and quality of life in eligible patients.